Roadmap for digital solutions
What are digital solutions?
Digital solutions, or digital health technologies, are mobile applications, programs and software used in the healthcare system, which can be used alone or in combination with other products or services. Such solutions are being used throughout the healthcare system, starting from supporting people's well-being and lifestyle and finishing with complex diagnostic and treatment devices.
Why are digital solutions needed in healthcare?
The use of digital healthcare technologies in the healthcare system, as well as in the social system, provides a way to ensure the sustainability of society in a situation of aging population, lack of health care professionals, and insufficient resources. Solutions can help people take more responsibility for their health, maintain and improve their health and quality of life, influence health-related behaviour, but also make processes more efficient, save time for healthcare professionals and save healthcare costs.
Why is it necessary to evaluate digital solutions?
Any evaluation and verification of the quality of applications on a standardized basis confirms the quality and reliability of digital solutions to the users of the solutions and those who recommend or finance them.
Due to the rapid growth of digitalization, in many countries, there has been an accelerated development of regulations and legal frameworks that would enable digital healthcare technologies to be evaluated and/or financed on a standardized basis. For example, Germany, Belgium and the United Kingdom have established their own frameworks.
At the same time, different frameworks and funding models are different even within Europe. For example, some countries have initially selected for evaluation and funding only mobile applications that qualify as medical devices, while others evaluate solutions more broadly, including both preventive and more complex applications that use, for example, robotics and artificial intelligence capabilities.
What is Estonia's experience with evaluating digital solutions in healthcare, and where did the idea of a digital solutions guide come from?
Over the past 20 years, Estonia has been purposefully digitizing the healthcare system by involving various companies, professional associations and medical institutions in this activity, who have led the digitization of one or another field. The Health Insurance Fund has been working on the evaluation of the effectiveness of digital solutions and the mapping of funding opportunities since 2019. In the same year, the Health Insurance Act was amended and an innovation fund was created, the funds of which can be used for the purpose of developing the healthcare system, to support the development of services potentially useful to the healthcare system and the evaluation of their impact.
In 2021, the Health Insurance Fund organized a competition for pilot projects of remote services, with the aim to speed up the introduction of useful remote services, test the compensation of such solutions and evaluate the impact of innovative solutions. During the competition, the organizers realized that before evaluating the evidence-based state and economic benefit of digital solutions, it is necessary to verify their efficiency and security. This can be assured by registering a digital solution as a medical device. In addition, the aspects related to information security and data protection needs to be evaluated. In order to make a reimbursement decision, the evidence-based data of the digital solution impact need to be compared with the current practice. To obtain an overview of the aspects that need to be evaluated and to find out the vision of market participants, , in the fall 2021, TalTech organized workshops with industry participants on behalf of the Health Insurance Fund and prepared a report that emphasized the need to systematically continue developing the field. Led by the Health Insurance Fund, a digital solutions guide was prepared in cooperation with our partners.
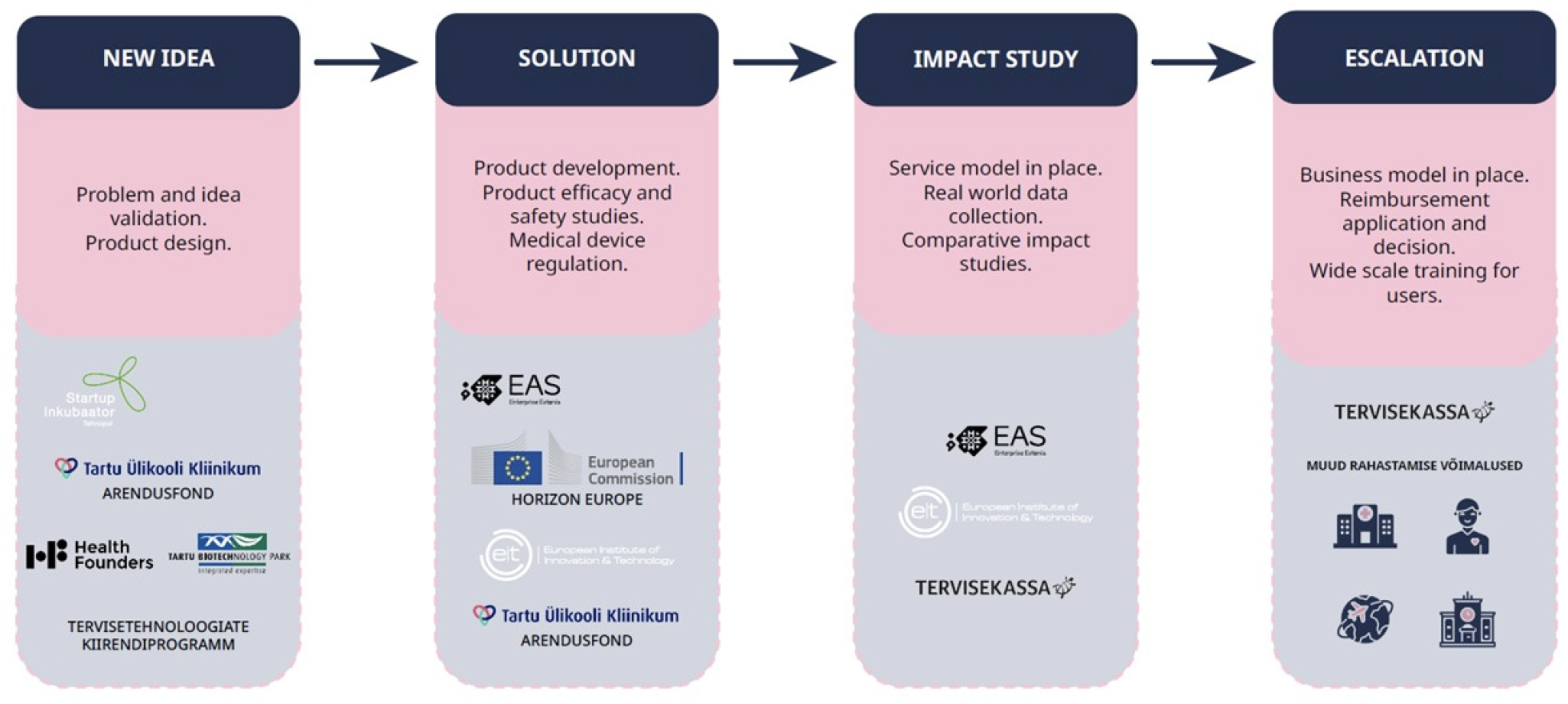
Innovation stages and funding measures of digital solutions
The development of digital solutions is a continuous process. Different stages have different goals and can be implemented with funding from different programs and sources. Innovation stages of digital solutions and their connection with some possible funding measures are described in the figure below.
Definitions and abbreviations
Digital solution. Digital health technology or digital solution is a mobile application, program or software that is used in the healthcare system. A digital solution can be used alone or in combination with other solutions or products (for example, physical medical devices and diagnostic assays).
Digital solution manufacturer. A company that produces, develops and sells digital solutions. Digital solutions manufacturers can be companies operating in various fields, including IT and pharmaceutical companies, as well as healthcare institutions.
Remote service. According to the European Commission, remote healthcare services or telemedicine can be defined as the provision of healthcare services at a distance, through the use of ICT. It involves secure transmission of medical data and information, through text, sound, images or other forms needed for the prevention, diagnosis, treatment and follow-up of patients.
Medical device. A wide range of different instruments, apparatus, equipment, reagents and also software used in healthcare and medicine. Medical devices include bandages, CT scanners, breast implants and pacemakers, as well as dentures, aids for the disabled and pregnancy tests. Based on their functionality, various digital solutions that can be used alone or in combination with some physical device can be classified as software based medical devices. The official definition of a medical device can be found in the European Union Medical Device Regulation (EU) 2017/745.
MDR (Medical Device Regulation). European Union Medical Device Regulation (EU) 2017/745.
IVDR (In-vitro Diagnostic Medical Devices Regulation). European Union In-vitro Diagnostic (EU) 2017/746.
Notified body. A conformity assessment body who is involved in the conformity assessment procedure of the device by the manufacturer. Notified bodies carry out conformity assessment procedures based on the conformity assessment route, which is determined depending on the risk class of the medical device. If the procedure is successfully completed, the manufacturer is issued with the appropriate EC certificate of conformity, which gives them the right to place their devices on the market.
X-Road. A data exchange platform used in Estonia, which enables secure information retrieval and exchange between institutions. To exchange data, an X-Road user should share their data, and all other users can use this data under an agreement. Read more about the X-Road here.
RIHA. Administration system for the state information system that provides an overview of various information systems and their documentation.
Contact
If you have any questions about the digital solutions guide, health insurance databases or innovation fund, please let us know. Also, do not hesitate to contact us if you have ideas for applying for funding from other sources and need cooperation partners or a letter of recommendation for this purpose.
We will engage the right people and find an answer to your question!
If you have any questions, contact Kristin Kuusk: kristin.kuusk [at] tervisekassa.ee