Medicinal products
The Estonian Health Insurance Fund processes the applications for new and generic medicinal products submitted by the manufacturers of medicinal products. The EHIF with Ministry of Social Affairs ensures that all the necessary medicinal products are put on the list of medicinal products distributed at a discount in timely manner on quarterly basis.
Medicinal products distributed at a discount
The establishment of a discount to a medicinal product is based on the following criteria:
- the need for a medicinal product, including the financial accessibility of a medicinal product distributed without a discount or with a lower discount rate;
- proven efficacy and safety of a medicinal product;
- the cost-effectiveness of a medicinal product (price and efficiency ratio, justification of price level);
- the existence of alternative medicinal products or treatments;
- possibilities for misuse and overuse of the medicine and their consequences;
- possibilities for providing the rational use of a medicinal product and a reliable prognosis of its retail sale volume;
- compliance with the financial means of health insurance, including the principle that the expenses incurred to provide medicinal benefits must not exceed 20 per cent of the expenses prescribed for health care benefits in the annual health insurance budget.
Amendment of the list of medicinal products distributed at a discount
The list of medicinal products distributed at a discount will be amended regularly, once per quarter (on 1 January, 1 April, 1 July and 1 October), simultaneously with the adjustment of reference prices.
Application for a new active substance to be added to the list of medicinal products distributed at a discount will be made by manufacturers of medicinal products who plan to launch a new product on the market and who have the most information about the efficiency, safety, and intended price level of the new medicinal products. Price negotiations will be held with them. Any interested party, including manufacturers of medicinal products and associations of specialised medical care specialists, can make applications with respect to the prescription of medicinal products that have already been entered to the list of medicinal products distributed at a discount.
The submission and processing of applications is described in the regulation of the Minister of Social Affairs: Procedure for drafting and amendment of a list of medicinal products of the Estonian Health Insurance Fund and the content of criteria establishing the list and evaluators of compliance with the criteria
In cases where the amendment of the list of medicinal products distributed at a discount might bring along important changes in the use of medicinal products and the financial obligations of the Estonian Health Insurance Fund, the Committee for Medicinal Products is included in the processing of applications.
The Committee for Medicinal Products has eight members – the representatives of the Estonian Medical Association, the Family Physicians Association of Estonia, the Estonian Chamber of Disabled People, the Estonian Patients Union, the State Agency of Medicines, the Estonian Health Insurance Fund, the Institute of Family Medicine and Public Health of the University of Tartu, and the Ministry of Social Affairs. The committee is an advisory body to the EHIF and the minutes of the discussions of the committee are public. The meetings of the committee are held at an average of every two months.
Pharmaceuticals
Reimbursement of pharmaceuticals
Discount rates for pharmaceuticals
EHIF reimburses to a certain extent for prescription medicines, the effectiveness of which has been previously thoroughly assessed and therefore they have been included in the list of reimbursed pharmaceuticals. These pharmaceuticals are available at a 50, 75, 90 or 100% discount. The highest discount rates are available for principal pharmaceuticals needed to treat serious and chronic diseases, or for certain groups of the population (old-age and incapacity pensioners).
The buyer has to pay a prescription fee of 3.5 euros for each prescription medicine.
The EHIF discount is calculated on the remaining part of the price according to the prescribed percentage. This means that the patient has to pay for the part exceeding the discount amount.
If there are many pharmaceuticals with the same active ingredient by different manufacturers on the market, a reference price is set for the whole group of pharmaceuticals with the same active ingredient, and the EHIF discount is calculated on this reference price. For example, if a pharmacy has a selection of medicines with the same active ingredient at the price of 5.60, 8.30, 10.50 and 12.80, the price of the second cheapest medicine (8.30 in this example) is used as the reference price and the EHIF discount is calculated on this amount. If the buyer prefers a more expensive medicine, then in addition to the basic rate (3.5 euros) and mandatory contribution, he/she must also pay for the part exceeding the reference price. Therefore, it is worth asking your pharmacist which medicine with the same active ingredient is sold at better price.
Reimbursement principles and list
The EHIF’s list of reimbursed pharmaceuticals established by a regulation of the Minister of Social Affairs and it is amended four times a year. The list includes pharmaceuticals that have a valid marketing authorization in Estonia and are subject to discount when purchased in a pharmacy.
If there are many pharmaceuticals with the same active ingredient by different manufacturers on the market, a reference price is set for the whole group of pharmaceuticals with the same active ingredient, and the EHIF discount is calculated on this reference price (100%, 75% or 50%).
To ensure stable marketing of pharmaceuticals and to prevent price increases, EHIF signs pricing agreements with pharmaceutical companies. Pricing agreements are signed for pharmaceuticals that are the only ones of their kind (with the same active ingredient and route of administration) and are included in the list of reimbursed pharmaceuticals. A pricing agreement is signed also for those pharmaceuticals that are in their price group cheaper than or equal to the reference price.
Discount rates and calculation
Only those pharmaceuticals that are indicated for the treatment or alleviation of diseases specified in the regulation of the Government of the Republic will be entered in the list of reimbursed pharmaceuticals.
100% discount
Patient pays the deductible of 3.50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 100% of the remaining price of pharmaceutical.
100% discount rate applies to pharmaceuticals indicated for the treatment of serious, life-threatening or epidemic diseases or the disease that cause severe pain. In addition, the 100% discount applies to all patients under 4 years of age. Please note! This does not apply to the prescription of iron prelates and food mixtures, in which case the conditions for age and diagnosis given separately in the list of medicinal products apply.
90% discount
Patient pays the deductible of 3.50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 90% of the remaining price of the pharmaceutical.
In some cases, the buyer of a pharmaceutical sold at 75% discount can use the 90% discount rate instead. The 90% discount rate applies to pharmaceuticals prescribed for children aged 4-16, persons receiving an incapacity for work or old-age pension and insured persons aged 63 and older.
75% discount
Patient pays the deductible of 3.50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 75% of the remaining price of the pharmaceutical.
The 75% discount applies to pharmaceuticals that are primarily indicated for the treatment or alleviation of chronic diseases.
50% discount
Patient pays the deductible of 3.50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 50% of the remaining price of the pharmaceutical.
The 50% discount rate applies to all other pharmaceuticals entered in the list of reimbursed pharmaceuticals that are indicated for the treatment of diseases that are not directly life-threatening, severe or chronic, or are used when the patient does not have a specific diagnosis. A 50% discount rate usually applies in case of mild illnesses that normally do not require long-term treatment (such as antibiotics).
Discount rates and calculation of reimbursement
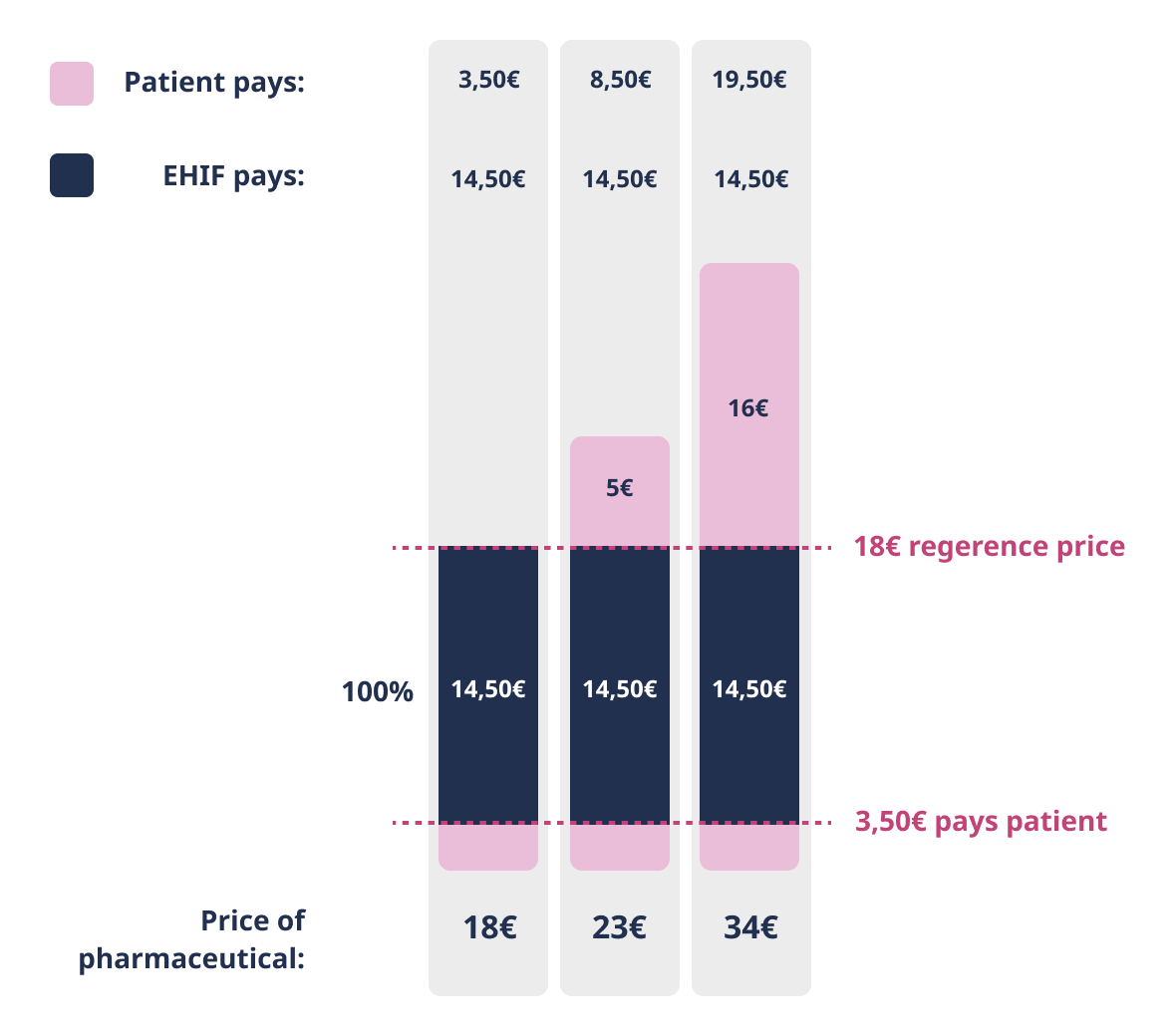
Patient pays the deductible of 3,50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 100% of the remaining price of the pharmaceutical.
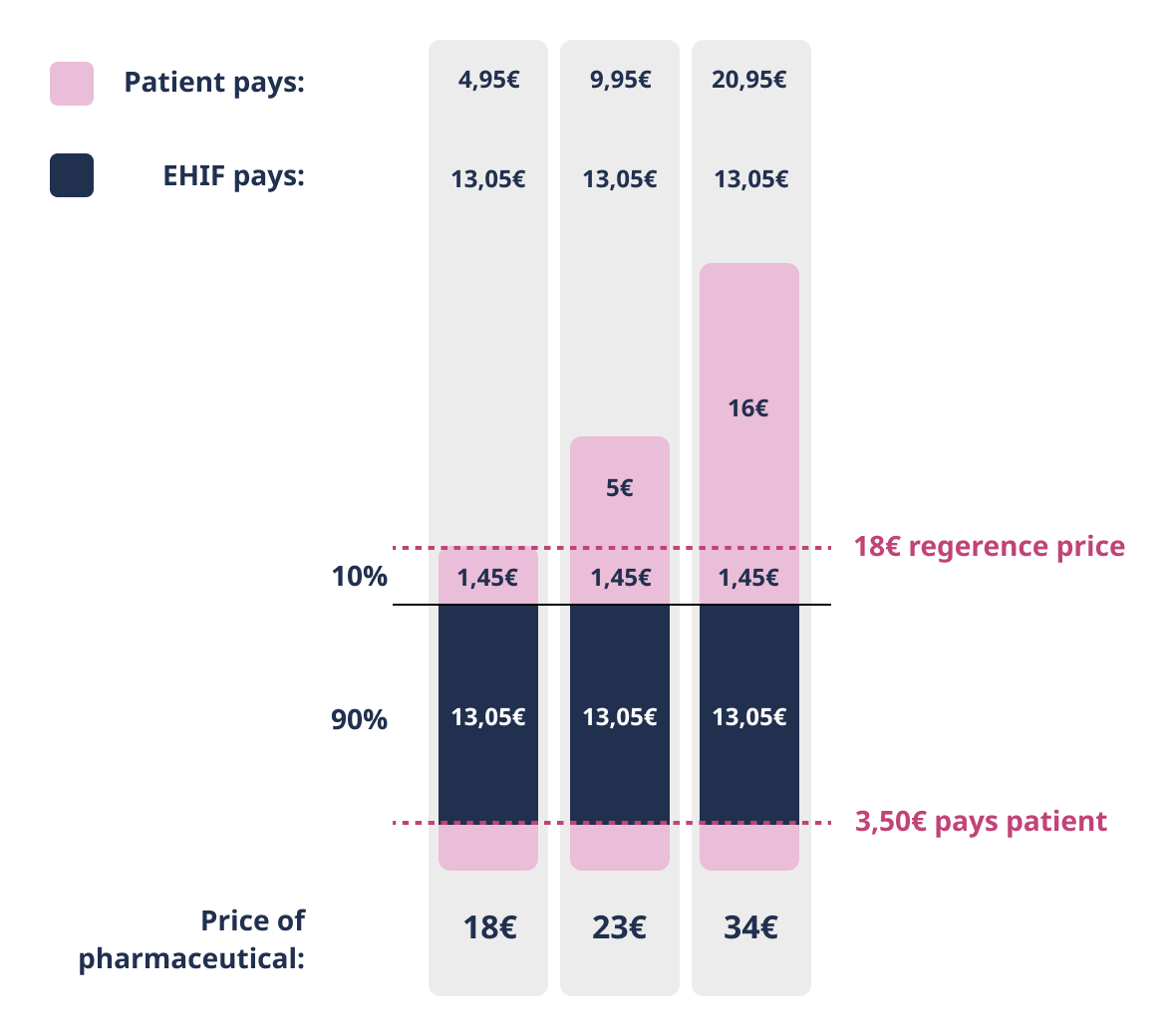
Patient pays the deductible of 3,50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 90% of the remaining price of the pharmaceutical.
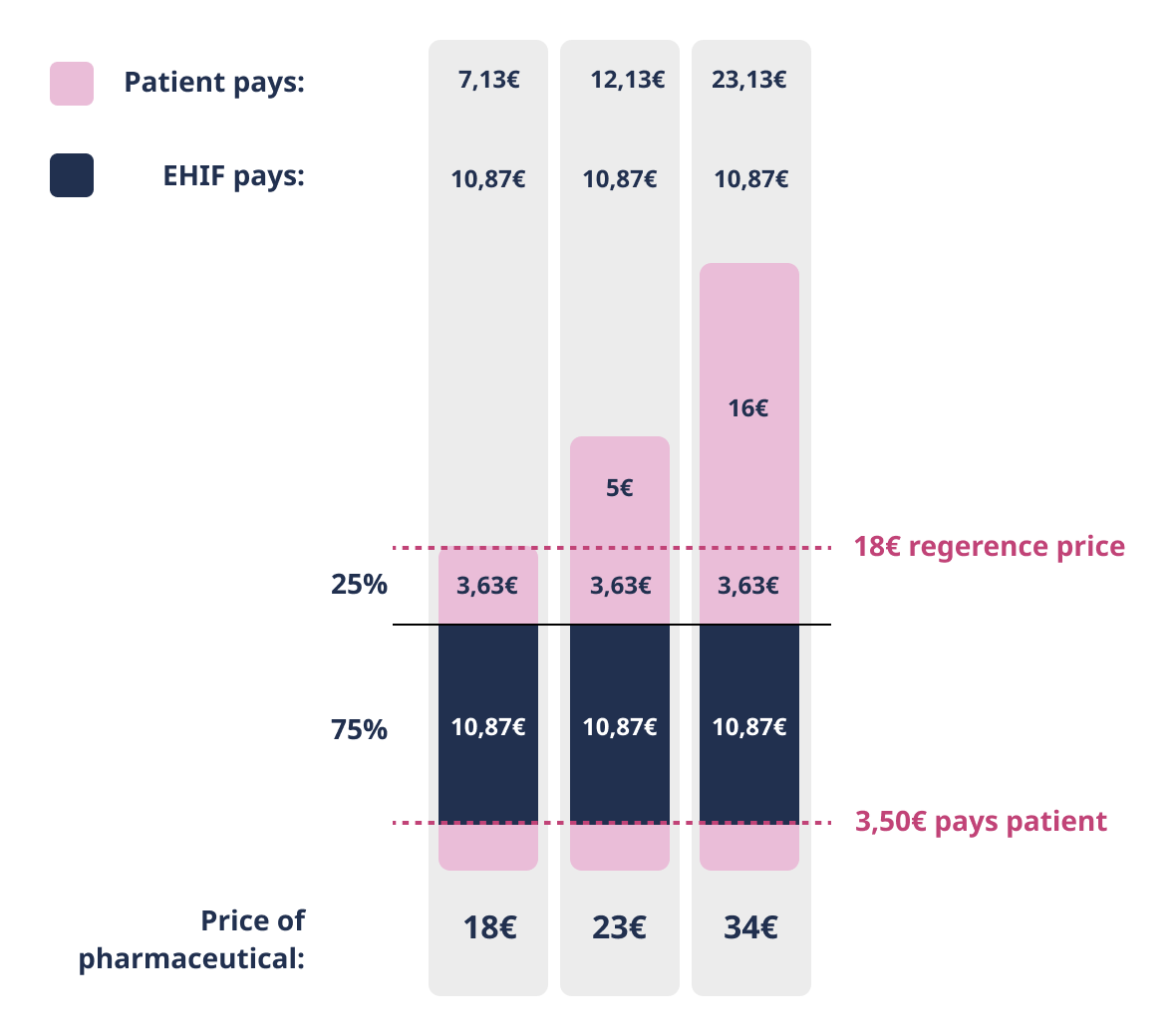
Patient pays the deductible of 3,50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 75% of the remaining price of the pharmaceutical.
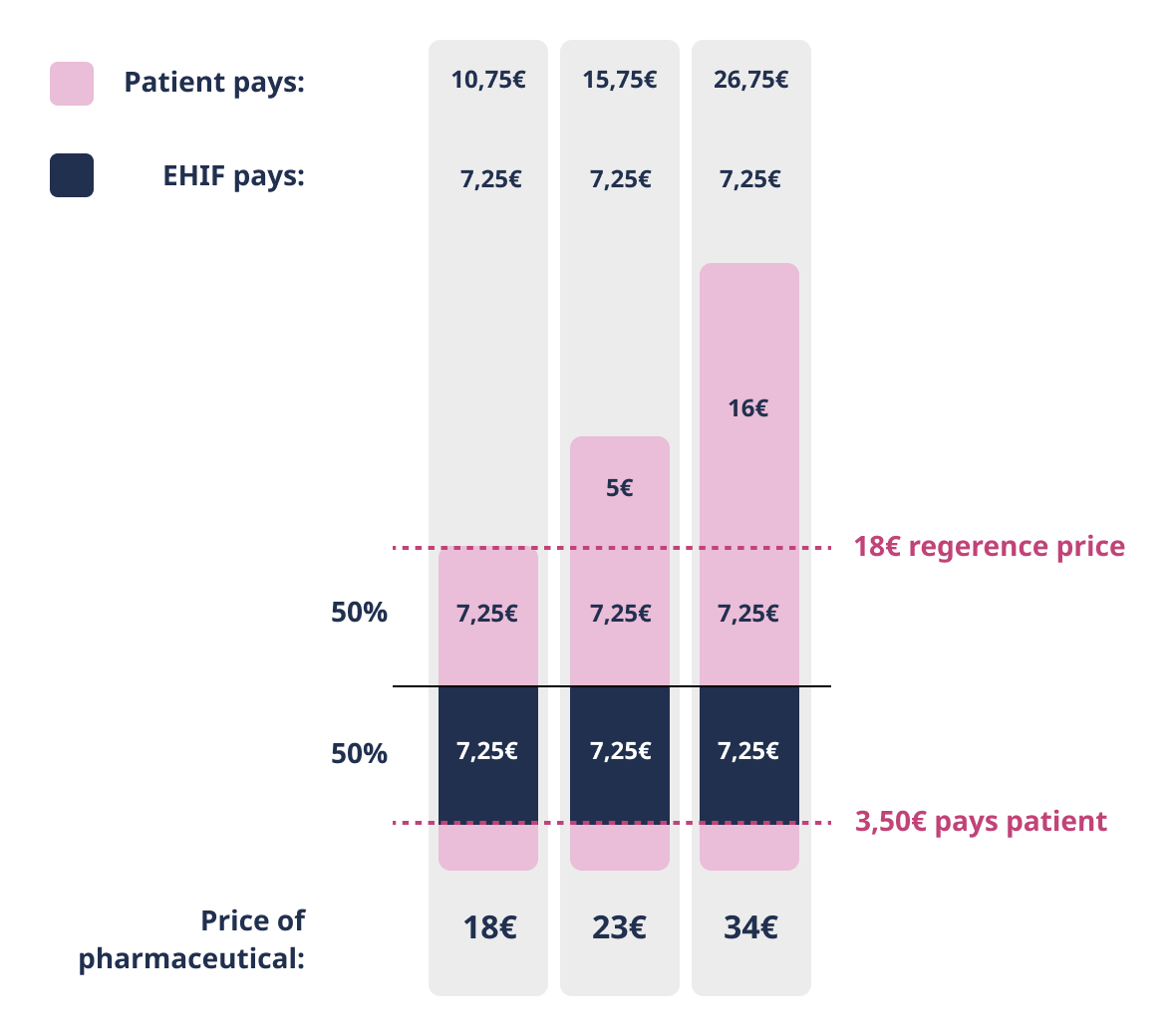
Patient pays the deductible of 3,50 euros and the amount exceeding the reference price or agreed price. EHIF reimburses 50% of the remaining price of the pharmaceutical.
More information:
- the list of reimbursed pharmaceuticals that is amended four times a year. The list includes medicines that have a valid marketing authorization in Estonia and are sold to the patient at a discount.
- reference prices of pharmaceuticals that are imposed on pharmaceuticals where there are several similar pharmaceuticals with the same active substance and route of administration in the list of reimbursed pharmaceuticals.
- pricing agreements that are signed to avoid price increases for medicines.
- information on the list of medicinal products, reference prices, pricing agreements.
- See information about the discount rates for all your prescriptions on the state portal: eesti.ee -> Home -> E-services -> For citizens -> Health and health protection -> Recipes
Additional benefit for pharmaceuticals
Supplementary benefit for medicinal products and medical devices (in effect from 1 January 2025
People who spend more than average on medicinal products and medical devices get a greater discount from pharmacies and companies selling medical devices. In addition to the normal discount on medicinal products and medical devices, there is a supplementary benefit for medicinal products and medical devices (hereinafter ‘the supplementary benefit’).
The supplementary benefit is paid to insured persons who pay at least 100 euros per calendar year for discounted prescriptions (not including the amount exceeding the maximum price).
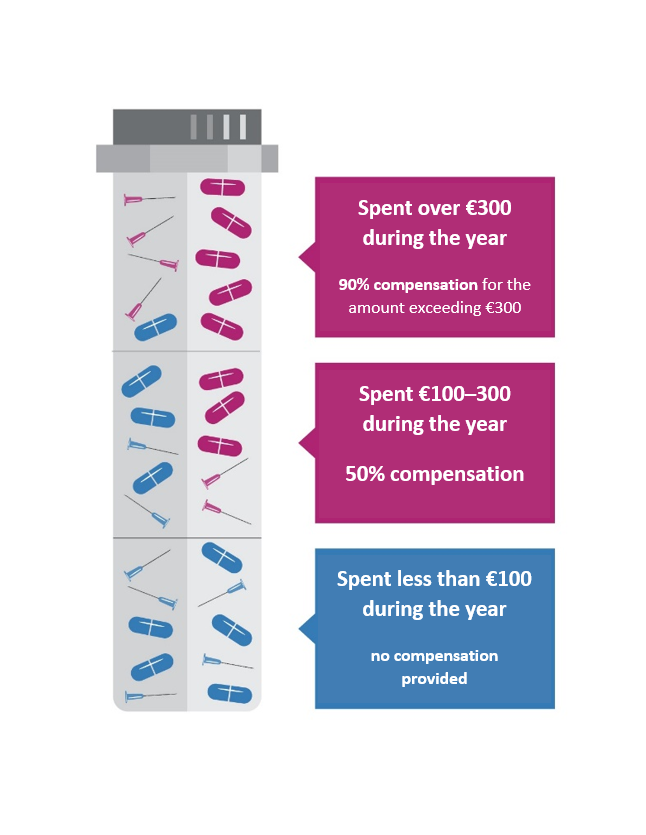
How is the supplementary benefit calculated?
The supplementary benefit applies to expenses made on medicinal products with an EHIF discount and on medical devices. This means that expenses made on over-the-counter medicines (non-prescription drugs) and prescription drugs that do not qualify for the EHIF discount are not taken into account. The benefit of the Health Insurance Fund also does not apply to discounted technical aids, which are reimbursed by the Social Insurance Board.
The supplementary benefit is calculated on the basis of expenses accrued in a calendar year. A prescription fee of €3.5 that applies when buying medicinal products is also taken into account.
The following principles apply to the reimbursement of medicinal products and medical devices purchased with discount prescriptions:
1. if the amount remains below 100 euros per year, no supplementary benefit is paid
2. if the amount is 100–300 euros per year, 50% of the amount exceeding EUR 100 will be reimbursed
3. if the amount exceeds 300 euros per year, 90% of the amount exceeding EUR 300 will be reimbursed
For example, if a person has spent 250 euros on medicinal products and medical devices, they will receive a discount of 75 euros from the Health Insurance Fund. In cases of expenses exceeding EUR 300 euros, the Health Insurance Fund will reimburse 90% of the out-of-pocket cost of the medicinal product or medical device. For example, if a person has spent 500 euros, they will receive a discount of 280 euros.
Calculation of the supplementary benefit for medicinal products and medical devices
1. First, the initial amount of the supplementary benefit for medicinal products and medical devices for the calendar year is calculated:
| Base amount of the supplementary benefit for medicinal products and medical devices. Please note! This amount is always lower than the amount spent on discount prescriptions. | = | The amount spent on discount medicinal products and medical devices. The costs of over-the-counter medicines, technical aids of the Social Insurance Board, and non-discount medicinal products and medical devices will not be taken into account. | - | The part of the price of medicinal products and medical devices that exceeds the reference price or the agreed price. |
2. If the base amount of the supplementary benefit for medicinal products and medical devices is known, the supplementary benefit for medicinal products and medical devices will be calculated as follows:
| The amount spent on medicinal products and medical devices | The Health Insurance Fund will reimburse | Example |
|---|---|---|
| Less than 100 euros | The supplementary benefit is not calculated | For example, if a person purchases a number of discount medicinal products and medical devices in a calendar year and the total out-of-pocket costs for these purchases amount to 120 euros, of which the unavoidable out-of-pocket costs are 80 euros (i.e. the person has also bought products that are more expensive than the reference price), the supplementary benefit will not be calculated. |
| 100 to 300 euros | 50% of the amount exceeding 100 euros | For example, if a person purchases a number of discount medicinal products and medical devices in a calendar year and with this purchase, the base amount of their personal supplementary benefit will increase to 105 euros, then they will be granted a supplementary benefit of 50% for the 5 euros exceeding 100 euros, and the person will pay 2.5 euros less at the pharmacy. If a person has spent 250 euros on medicinal products and medical devices, we will deduct 100 euros from this amount for the supplementary benefit, which is the minimum payment threshold, and make a calculation of 150 × 50% = 75 euros. |
| More than 300 euros | 90% of the amount exceeding 300 euros | If the base amount of the supplementary benefit was 300 before the purchase and a medicinal product or medical device was purchased which raised the base amount to 320, the supplementary benefit will be paid at 90% of the amount exceeding 300 euros (90% of 20 = 18 euros) and the person will pay 18 euros less at the pharmacy or the company selling medical devices. It is essential to keep in mind that from 100 to 300 euros, the 50% benefit has already been received from previous purchases. However, if the base amount was 90 euros before the purchase and an expensive medicinal product or medical device was purchased which raised the base amount to 500 euros, the person will receive a supplementary benefit of 280 euros (i.e. 50% of the difference of 100 euros and 300 euros (i.e. 200 euros, of which 50% is 100 euros) + 90% of the amount which exceeds 300 euros (90 % of 200 = 180 euros) |
Please note! The amount exceeding the reference price or the agreed price (the avoidable out-of-pocket cost) will be deducted from the amount spent on medicinal products and medical devices before the calculation of the supplementary benefit. If there are many medicinal products with the same active substance in the market from different manufacturers, a reference price for the whole group of medicines with the same active substance will be established, from which the EHIF discount will be calculated. For example, if a pharmacy has a selection of medicines with the same active substance at a price of 5 euros, 7 euros, and 9 euros, then the reference price will generally be the price of the second most affordable medicine (7 euros in this example) and the benefit percentage of the Health Insurance Fund will be calculated from this amount. If the purchaser of a medicinal product prefers a more expensive medicine, the part exceeding the reference price must be paid at the pharmacy in addition to the established out-of-pocket cost (2 euros in this example).
A similar principle applies to medical devices. If there are several comparable devices in the list of medical devices in the same medical device reference price group, the reference price is the reference price of the second most affordable medical device, from which the benefit percentage of the Health Insurance Fund is calculated. For example, if medical device sellers offer different glucometer test strips at 15 euros, 16 euros, and 19 euros, the reference price is set at 16 euros and the benefit percentage of the Health Insurance Fund is calculated from this amount. If the purchaser prefers a more expensive medical device, the part exceeding the reference price must be paid in addition to the established out-of-pocket cost (3 euros in this example).
Reimbursement of pharmaceuticals related to artificial insemination
From 2018, in vitro fertilization (IVF) and embryo transfer services and pharmaceuticals are fully financed by EHIF. Previously, the Ministry of Social Affairs paid for these pharmaceuticals and services. In addition, from 2018, you do not have to apply for reimbursement for IVF pharmaceuticals after you have purchased them.
Women up to the age of 40 (incl.) are entitled to a 100% discount on the pharmaceuticals necessary for in vitro fertilization immediately at a pharmacy when buying them. At the pharmacy, they only have to pay the prescription fee, which is 2.5 euros, and the part exceeding the maximum price of the reimbursable pharmaceutical, provided that the pharmaceutical is more expensive than the reference price listed by EHIF. Ask your doctor for more information about different pharmaceuticals and in vitro fertilization.
The pharmacy offers a 100% discount for pharmaceuticals that contain the following active ingredients:
- follitropin alpha;
- follitropin beta;
- ganirelix;
- goserelin;
- triptorelin;
- cetrorelix;
- lutropin;
- corifollitropin;
- chorionic gonadotropin alpha.
|
Reimbursable medicines |
ATC code |
Active substance |
|---|---|---|
|
PREGNYL inj subst 5000 IU vial 1 ml solvent N1 |
G03GA01 |
chorionic gonadotropin |
|
BEMFOLA 150 IU/0.25 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 225 IU/0.375ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 300 IU/0.5 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 450 IU/0.75 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 75 IU/0.125 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 300 IU/0.5 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 450 IU/0.75 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
BEMFOLA 900 IU/1.5 ml solution for injection in a pre-filled pen N1 |
G03GA05 |
follitropin alpha |
|
GONAL-F powder and solvent for solution for injection 75 IU N1 |
G03GA05 |
follitropin alpha |
|
OVALEAP 300 IU/0.5 ml solution for injection N1 |
G03GA05 |
follitropin alpha |
|
OVALEAP 450 IU/ 0.75 ml solution for injection N1 |
G03GA05 |
follitropin alpha |
|
OVALEAP 900 IU/1.5 ml solution for injection N1 |
G03GA05 |
follitropin alpha |
|
PUREGON solution for injection 300 IU 0.36 ml N1 |
G03GA06 |
follitropin beta |
|
PUREGON solution for injection 50 IU 0.5 ml N5 |
G03GA06 |
follitropin beta |
|
PUREGON solution for injection 900 IU 1.23 ml N1 |
G03GA06 |
follitropin beta |
|
LUVERIS powder and solvent for solution for injection 75 IU N1 |
G03GA07 |
lutropin alpha |
|
OVITRELLE solution for injection in a pre-filled pen 250mcg/0.5ml N1 |
G03GA08 |
chorionic gonadotropin alpha |
|
ELONVA solution for injection 100mcg/0.5ml N2 |
G03GA09 |
corifollitropin alpha |
|
ELONVA solution for injection 150mcg/0.5ml N1 |
G03GA09 |
corifollitropin alpha |
|
ORGALUTRAN solution for injection 0.25 mg/0.5 ml 0.5 ml N1 |
H01CC01 |
ganirelix |
|
CETROTIDE powder and solvent for solution for injection 0.25 mg N1 |
H01CC02 |
cetrorelix |
|
RESELIGO implant in a pre-filled syringe 10.8 mg N1 |
L02AE03 |
goserelin |
|
RESELIGO implant in a pre-filled syringe 3.6 mg N1 |
L02AE03 |
goserelin |
|
ZOLADEX implant 3.6mg N1 |
L02AE03 |
goserelin |
|
DIPHERELINE 11.25 MG prolonged suspension powder 11.25mg N1 and solvent N1 |
L02AE04 |
triptorelin |
|
DIPHERELINE 3.75 MG prolonged suspension powder 3.75mg N1 and solvent N1 |
L02AE04 |
triptorelin |
*When calculating the benefit, the patient's own contribution of 2.50 euros of the discount prescription is not taken into account.

