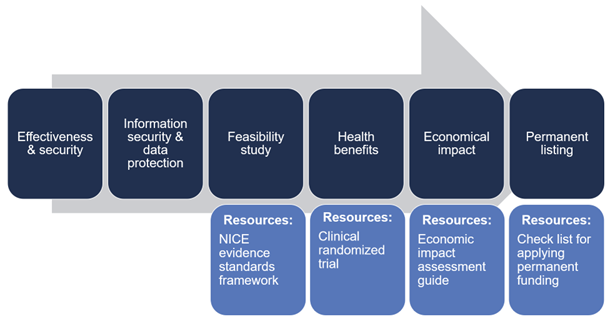
Impact assessment studies
Investigating the impact of new (digital) service models is still an evolving field. Internationally, there are no unified standards for researching healthcare technologies to measure the impact of a new solution most effectively. Digital solutions are products that are constantly evolving and highly dependent on end-users. For example, software can be continuously modified based on clinical performance and end-user input. Additionally, interventions can be continuously adjusted according to individual needs and changing circumstances. However, due to the flexibility of digital solutions, it is very difficult to measure their clinical impact and effectiveness.
In healthcare, the gold standard is a clinical randomized trial, but the structure of such a study may make it challenging for service models containing digital solutions to demonstrate their full value. It is important for the Health Insurance Fund to ensure that individuals receive the best possible treatment, considering the evidence-based nature of the treatment, medical effectiveness, and cost-effectiveness. Until there are specific agreements and good examples of conducting studies on digital solutions, we rely on the best practices of randomized clinical trials for research on digital solutions as well.
In designing the impact studies of pilot projects, the NHS’s NICE framework was used as a guiding material. The experience with the NICE framework showed that the framework is a useful tool for conducting initial feasibility studies, but to obtain permanent funding, one study alone is not sufficient. More research is needed to obtain stronger evidence.
The figure illustrates various stages. More information can be found here.